 |
Carol Hoppe
MedLucid Solutions |
In the
2019 Final Rule, CMS confirmed the Jan. 1, 2020, implementation date for Clinical Decision Support (CDS), also known as Medicare Appropriate Use Criteria (AUC) consultations, when ordering outpatient advanced imaging studies such as CT, MRI, PET and nuclear medicine. Furnishing providers (radiologists and imaging centers) must document AUC consultations by the ordering provider on professional and technical claims for Medicare fee-for-service beneficiaries in the outpatient setting.
CDS consultation will be required when imaging is provided in the following outpatient settings:
- Physician offices.
- Hospital outpatient departments (including non-emergency conditions in the ED).
- Ambulatory surgery centers.
- Independent Diagnostic Testing Facilities (IDTFs) – added in 2019
Since July 1, 2018, ordering providers using CDS may receive credit for two MIPS categories (Improvement Activities and Advancing Care Information). During this voluntary reporting period, furnishing providers can report HCPCS modifier QQ for AUC consultations.
Starting in January 2020, ordering providers will be required to consult AUC through a Clinical Decision Support Mechanism (CDSM). “A CDSM is an electronic portal for accessing AUC during the patient workup. CDSMs will provide ordering providers with a determination of whether the order adheres or does not adhere to AUC, or if there is no AUC applicable,” according to
MLN Matters.
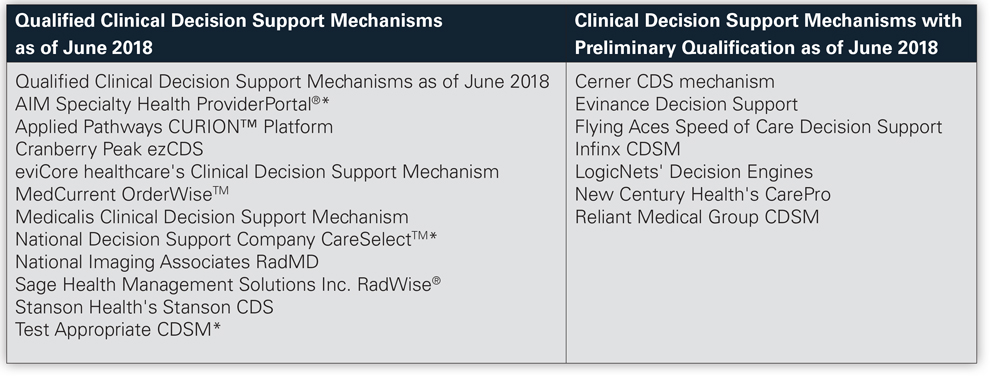
Current qualified CDSMs, listed below,
are identified on the CMS website.
While there are no financial penalties in 2020 during the “educational and testing” period, ordering providers who attest to CDS consultation are incentivized by continuing to earn credit under MIPS. Furnishing providers will begin to see claim denials starting on Jan. 1, 2021, if the AUC consultation G codes (yet to be developed by CMS) are not reported. This will have a significant financial impact on radiologists who furnish and interpret these tests.
Exceptions
There are three hardship exceptions that apply to ordering providers:
- EHR or CDSM vendor issues.
- Insufficient internet access.
- Extreme or uncontrollable circumstances.
Inpatient services and suspected emergency medical conditions are also exempt. These hardships will be self-reported with a modifier, yet to be assigned by CMS.
While the financial burden appears to fall entirely on the furnishing providers, this also has implications for ordering providers. Radiologists and imaging centers may begin returning or denying orders as soon as Jan. 1, 2020, but will be forced to do so once the financial impact is recognized in 2021. CMS will also be looking at eight priority areas that will identify ordering providers who are not compliant with AUC guidelines.
- Coronary artery disease (suspected or diagnosed).
- Suspected pulmonary embolism.
- Headache (traumatic and non-traumatic).
- Hip pain.
- Low back pain.
- Shoulder pain (to include suspected rotator cuff Injury).
- Cancer of the lung (primary or metastatic, suspected or diagnosed).
- Cervical or neck pain.
Up to 5 percent of ordering providers will be identified as outliers, who will then be required to obtain preauthorization when ordering imaging for Medicare beneficiaries. CMS is expected to address this further in 2022 or 2023.
It will be important for all physicians to begin developing an implementation strategy this year to allow time to test systems, train providers and staff and roll out new processes. CMS will allow clinical staff to consult CDS on behalf of ordering providers to ease the administrative burden. To qualify, clinical staff must be working under the direction of the ordering provider and have sufficient clinical knowledge to consult CDS and communicate results back to the ordering provider. This only applies to staff within the ordering provider's office; it does not include radiology staff or radiologists.
Stay tuned for more information on this topic as it becomes available. Talk to your EHR vendor now to find out what its strategy is for addressing CDSM!
If you have questions, contact ISMA at (317) 261-2060.
References:
2019 Final
Rule, Federal Register / Vol. 82, No. 219 / Wednesday, November 15, 2017 /
Rules and Regulations
Appropriate Use Criteria for
Advanced Diagnostic Imaging – Voluntary Participation and Reporting Period -Claims Processing Requirements – HCPCS Modifier QQ
Clinical Decision Support Mechanisms